
Van GoghTM
Current techniques for tissue evaluation take time, damage samples, and can produce inadequate assessments, resulting in prolonged procedures and the need for additional biopsies. These setbacks can delay diagnoses and impact patient treatment timelines. Aquyre Biosciences’ Van GoghTM addresses these challenges.
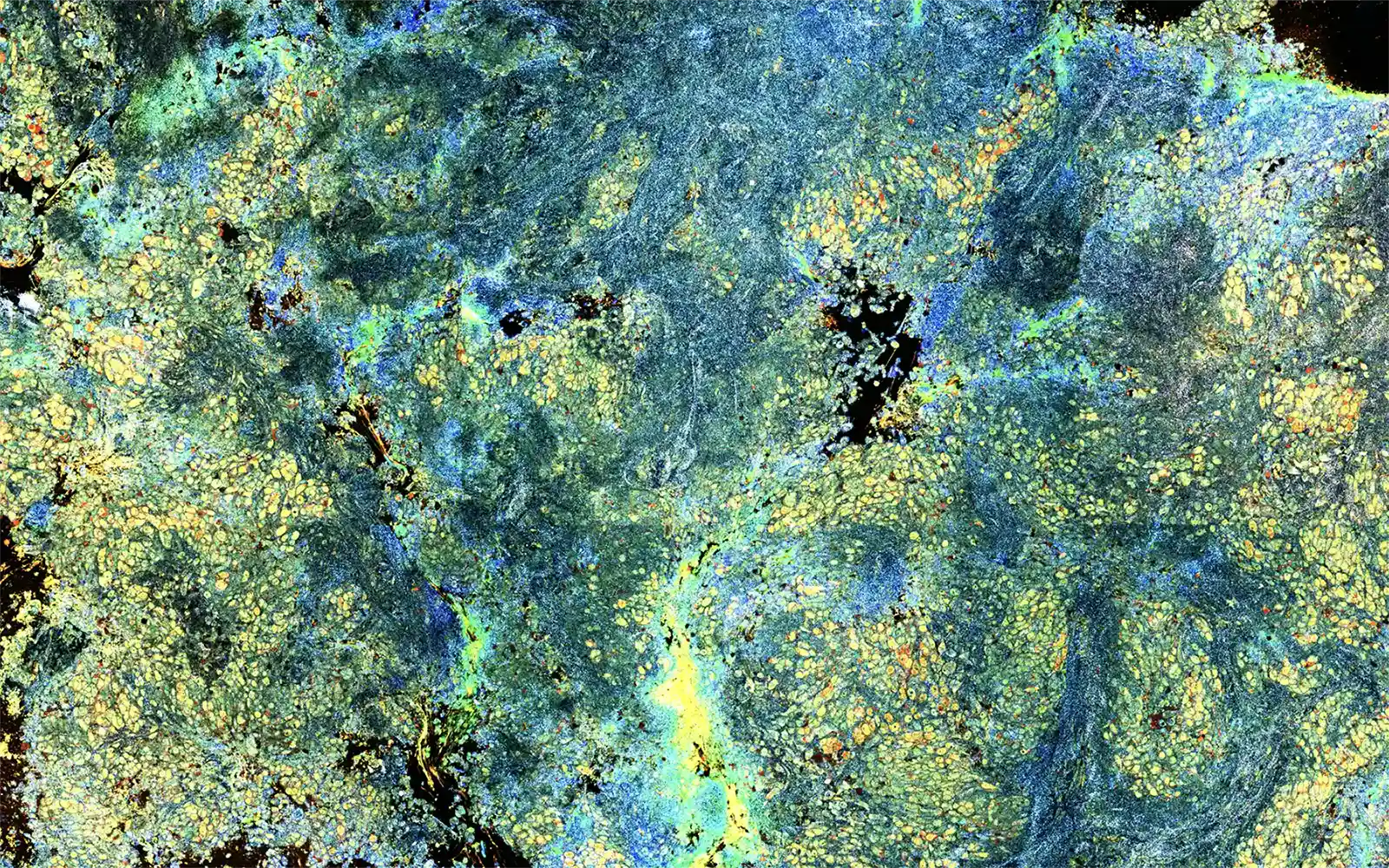
Powered by two proprietary technologies, including Dynamic Cell Imaging (DCI), Van Gogh can analyze any biopsy at a depth of 150μm below the tissue surface and measure intracellular and metabolic activity throughout. This process does not require any cutting or staining, leaving the sample unchanged for final pathology to accurately diagnose and perform next-generation sequencing (NGS). Using light, the cell shape, size, and activity are highlighted in a heatmap, making it easier to assess tissue adequacy.
Intended use: An FDA Class I in-vitro diagnostic medical device.
Van Gogh
Concordance with final path
Average time for results
Of sample is usable by pathology

Join The ROSE Revolution
Accelerate the timeline of diagnosis and improve confidence in yield by adding Van Gogh to your workflow.


